Industrial Process Emissions Policies
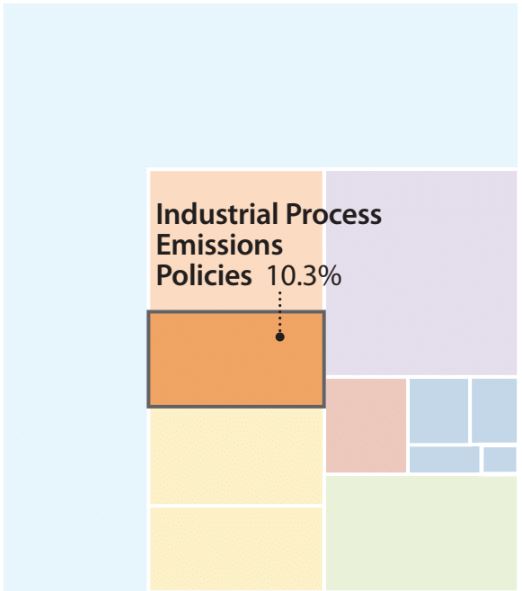
Potential emissions reductions from industrial process emissions policies.
Industrial process emissions encompass all the non-energy ways industrial production releases greenhouse gases into the air, from enteric fermentation in livestock to natural gas pipeline leaks. Process emissions include methane, nitrous oxide, and various fluorinated gases (F-gases) that contribute significantly to global warming. Industrial process emissions policies can contribute more than 10% of the cumulative global emissions needed to meet the two-degree Celsius target.
In 2016, process emissions generated 1.6 billion metric tons of CO2e in the U.S., representing 55% of all industry-related emissions and 24% of economy-wide emissions. In 2016, China generated 2.6 billion metric tons of industrial process emissions, representing 28% of its industry emissions and 20% of economy-wide emissions.
These differences illustrate how developed and developing economics must approach reducing process emissions in distinct ways when nations have more heavy industry, less energy-efficient industries, and a larger share of coal-fired electricity. Despite the variety of available techniques and technologies to cut emissions, no single technique or technology can reduce all process emissions.
Fortunately, a limited number of industrial processes are responsible for most process emissions: Seven sources of process emissions were responsible for nearly 90% of all process emissions globally in 2010.
This high emissions concentration in a small set of processes means a small number of technologies and strategies such as monitoring and reporting requirements, performance standards, carbon pricing, and financial and technical assistance can have an outsized impact on reducing process emissions.
Policy Description and Goal
Because most of the world’s process emissions are produced from a narrow range of activities, our efforts will be most efficient and will have a greater impact if we focus on nine specific types of emissions:
- Enteric fermentation and manure from livestock
- Methane leaks from natural gas systems
- Cement clinker production
- Greenhouse gases from soils, rice cultivation, and fertilizer use
- Methane produced in landfills
- Refrigerant use
- Methane leaks from coal mines
- Methane from wastewater treatment
- Metallurgical coke for iron and steel production.
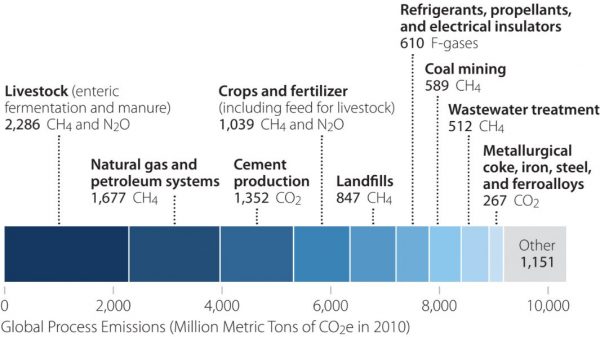
Nine processes dominate global process emissions. Source: U.S. EPA.
Techniques exist to tackle each of these types of emissions, and policies such as monitoring and reporting requirements, performance standards, carbon pricing, and financial and technical assistance can help achieve emission reductions from each of these processes. Examples of successful programs include the Montreal Protocol (which in 2016 was expanded to cover refrigerants) and methane reduction initiatives to promote the use of anaerobic digesters (discussed below) in California and Zimbabwe.
Livestock Measures
Animal husbandry produces greenhouse gas emissions through two mechanisms: the decomposition of manure and enteric fermentation. If manure is left unmanaged, bacteria that consume nutrients in the manure release methane as a byproduct of their metabolism.
The primary method of reducing these emissions is to process manure in anaerobic digesters, which convert the produced methane into electricity. This benefits the environment and provides power for farm or dairy operations. Anaerobic digesters are a commercialized technology, making them a promising method for achieving emission reductions from livestock. For example, in 2016 California passed legislation that will regulate greenhouse gas emissions from livestock beginning in 2024, and regulators are focusing on deploying anaerobic digesters as a means to hit the state’s target.
The other major source of livestock methane is enteric fermentation. Certain herbivores, called ruminants, have two stomachs. Plant matter chewed and swallowed by a ruminant is sent to the first stomach, where bacteria help to break down the food in a process called fermentation. Methane is released as a byproduct, which is then expelled by the animal, mostly by belching. The animal later moves the plant matter back to the mouth, re-chews it, and sends it to the second stomach, which leads onward to the rest of the digestive system. Ruminants convert more of the energy in their feed into methane than non-ruminant animals.
For example, a ruminant such as a cow may convert 5.5%–6.5% of the feed energy into methane, whereas that percentage is closer to 2.5% for horses and 0.6% for swine (both non-ruminants). Because of the large number of cattle in agriculture systems worldwide, the large amount of feed consumed by each cow, and the high methane conversion percentage for ruminants, cattle are responsible for most methane produced by enteric fermentation.
Various techniques have been proposed for reducing methane from enteric fermentation, such as varying the type, schedule, or quantity of food given to animals; including supplements in the food; or even vaccinating the animals so their immune systems attack methane-generating bacteria. None of these practices are yet well established or used commercially.
Finally, a more direct way to reduce emissions from both manure and enteric fermentation would be to reduce the number of animals (particularly ruminants) in the agriculture system by reducing demand for meat and dairy products.
Prevent Methane Leaks from Natural Gas and Petroleum Systems
Methane, a powerful greenhouse gas, is the primary component of natural gas. It is colorless and scentless. (The familiar smell of natural gas is an odorant that is added for safety reasons.) Natural gas can leak at any point in its economic lifecycle: from the wellhead where it is produced, pipelines and meters that carry the gas to customers, customer-owned pipes and valves behind the meter, or gas-fired appliances.
Natural gas leaks are difficult to eliminate. A single oil and natural gas field may have up to a million connections (e.g., joints between pipes, gaskets, valves), and if even a small percentage of them are not perfectly airtight, high-pressure gas can be forced out. Natural gas pipelines in cities can be decades old and buried underground, making detection and repair of leaks difficult. For example, the City of Boston loses $1 billion worth of natural gas each decade though leaks from its aging cast-iron pipelines.
Nonetheless, a variety of techniques can be used to detect and minimize methane leakage. Infrared video cameras can visualize methane plumes, and oil field operators may regularly survey their equipment with camera-equipped vehicles to check for leaks. Technology exists to capture the gas in the flowback from a newly drilled well, a process called green well completions.
And in the U.S., studies have found that a disproportionate amount of leaked methane from oil and natural gas production facilities comes from a small number of problem sites, providing a chance to reap outsized benefits from tackling these opportunities. In some cases, fixing leaks saves money, because the gas being lost to the atmosphere is the product being transported and sold to end users.
Cement Clinker Substitution
Cement is an essential component of concrete, one of the most important building materials used around the world. Clinker is a hard substance that composes 74%–84% of cement by weight, varying by world region, and is created by the breakdown of calcium carbonate (CaCO3), the main constituent of limestone rock, into lime (CaO) and carbon dioxide (CO2). The CO2 is released to the atmosphere, and the lime reacts with silica, aluminum, and other materials to become part of the clinker.
The main method by which cement clinker emissions can be mitigated is to reduce the share of clinker in cement. Other materials such as fly ash (a waste product from coal combustion) may be substituted for a portion of the clinker. However, there is a limit to how low the clinker percentage may be before the cement has undesirable structural properties. It is estimated that the clinker percentage may be reduced to as little as 70%.
Another way to reduce emissions is by improving building quality and longevity, particularly in developing countries where construction standards may be lower than in developed countries.
For example, in China, “the average period of time that . . . housing remains livable is a meager 35 years compared to a century or more in many developed countries,” and many buildings last no more than 20 years. If buildings, roads, and other infrastructure need to be rebuilt every few decades, this requires much more cement production and hence results in more process emissions than if buildings last a century.
Cropland and Fertilizer Management
Growing crops generates greenhouse gas emissions through the decomposition of organic matter in the soil and the application of fertilizer.
Each year, organic matter is added to the soil as crops grow, and it later decays, releasing CO2 and methane. Tilling (turning over and breaking up the soil) is often performed before planting, to loosen compacted soil and mix nutrients.
However, this process exposes organic matter to air and accelerates the release of emissions into the atmosphere. Tilling the soil less frequently or refraining from tilling altogether can allow more carbon to build up in the soil until the soil becomes saturated with carbon in about 20–25 years. However, reducing or eliminating tilling can reduce the productivity of some crops.
After the harvest each year, planting grass or a legume as a cover crop for the winter can help reduce the release of organic matter from soils. Also, a winter cover crop may allow less fertilizer to be used in the next season.
Reduced tillage and the use of winter cover crops must be continued for as long as the carbon storage is to be maintained. Should the cropland be returned to typical farming practices, the stored carbon will be released to the atmosphere.
Application of fertilizer results in N2O emissions, because only some of the nitrogen in the fertilizer is successfully taken up by the plants. There are techniques to increase the fraction of applied nitrogen that is used by plants, including improved fertilizer application methods and timing, reduction in the amount of fertilizer used, and the use of nitrification inhibitors. Nitrification inhibitors are chemicals that slow the conversion of ammonium (NH4+ in decomposed fertilizer) to nitrate (NO3–, a plant-available form of nitrogen), so that plants have time to capture more of the nitrate before it volatilizes or leaches away.
Additionally, several considerations are particular to the growing of rice. Around the world, most rice is grown in paddy fields, which are flooded with water periodically throughout the growing season. While a rice field is flooded, the soil is in an anaerobic environment, or deprived of oxygen. This allows microbes to produce methane as they ferment organic matter in the soil.
A key method of reducing methane emissions from rice cultivation is to reduce the amount of time the rice fields remain flooded. Draining a field halfway through the growing season or engaging in alternating wetting and drying greatly reduces methane emissions, and these practices may or may not decrease crop yields, depending on the soil conditions, climate conditions, wetting-and-drying technique, and rice cultivar.
However, these water management practices may not be possible in some areas that are naturally flooded, where farmers lack reliable control over irrigation systems, or where fields are not sufficiently level (because of the formation of wet and dry pockets).
Preventing Methane Leaks from Landfills
The decomposition of organic material under anaerobic conditions in landfills results in the generation of methane, which makes up roughly 50% of landfill gas. Rather than being allowed to reach the atmosphere, landfill gas can be harvested by drilling wells in the landfill and using a blower or vacuum system. This allows the gas to be collected at a central point, where it can be used to generate electricity (usually by powering an internal combustion engine) or may be used directly to replace another fuel, such as natural gas or coal.
Another way to reduce landfill methane emissions is to divert organic waste from landfills through techniques such as reducing food waste and composting (During composting, decomposition typically occurs in aerobic conditions, which produces much less methane than anaerobic decomposition.).
Substituting High Global Warming Potential Refrigerants
Fluorinated gases (F-gases) are chemicals used for a variety of industrial purposes. For example, F-gases are often used as a refrigerant, as a propellant in aerosol canisters, and as an electrical insulator in high-voltage transmission systems.
Many F-gases are replacements for ozone-depleting substances, chemicals that damaged Earth’s ozone layer and that were largely phased out as a result of the 1987 Montreal Protocol. Although the remaining F-gases used in industry today do not damage the ozone layer, many are powerful and long-lived greenhouse gases, so they contribute significantly to global warming.
Most F-gases that are produced eventually reach the atmosphere. For example, the refrigerant in an air conditioner may leak slowly, or it may be released when the air conditioner is scrapped at the end of its useful life. One way to reduce F-gas emissions is to better seal systems against leaks and to establish recycling or takeback programs for old products containing F-gases.
However, the best technique is to avoid creating F-gases in the first place by substituting different chemicals in industrial applications. The best choices are chemicals that neither contribute to global warming nor damage the ozone layer but still allow the system to operate at high efficiency.
Examples of environmentally friendly refrigerants include R-717 (ammonia), R-744 (CO2), R-1270 (propylene), R-290 (propane), R-600a (isobutane), and R-1150 (ethylene). Some of these alternatives come with their own dangers (e.g., propane is highly flammable), but different substitutes can be used for different applications to lower risks.
The 2016 Kigali amendment to the Montreal Protocol, discussed as one of the case studies in this section, mandates the phase-out of many F-gases and the substitution of less harmful refrigerants and propellants.
Controlling Methane Leaks from Coal Mining
As coal is formed underground over millions of years, methane is also formed in the coal seams. Coalbed methane refers to all of the methane that forms in these seams, whereas coal mine methane refers to the portion of that methane that would be released through mining activities.
Equipment may be used to capture the methane emitted from coal mines, particularly from degasification systems, which emit methane at a higher concentration than ventilation systems. Mine ventilation systems are the single largest source of methane emissions from coal mines, but the high air flow rate and low methane concentrations (less than 1%) make it difficult to capture and use this methane cost-effectively.
Captured coal mine methane can be put to economically productive uses. Captured coal mine methane is most often used for power generation, district heating, or boiler fuel, or it can be used on-site for coal drying, fueling mine boilers, or other purposes.
Finally, technologies that reduce the use of coal as an energy source (such as renewable energy sources) might allow for fewer coal mines and thereby reduce coal mine methane emissions.
Reducing Methane Emissions from Wastewater Treatment
Methane is produced from decomposing organic material, especially under anaerobic conditions. In developed countries, most wastewater is treated in aerobic conditions, so little methane is generated directly. However, the biosolids that remain after water treatment, if not managed properly, may produce methane. In developing countries, wastewater, if treated at all, is usually treated under anaerobic conditions and produces methane directly.
Biosolids may be processed in an anaerobic digester. (Anaerobic digesters are discussed earlier, in the “Livestock Measures” section, and later as a case study.) In developing countries without modern wastewater treatment facilities, the best solution is to construct centralized aerobic water treatment plants, if the population and infrastructure can support these facilities.
Otherwise, existing anaerobic lagoons may be retrofitted with covers and biogas capture systems, a simple and low-cost measure. Additionally, it is important to ensure staff are trained to maintain and efficiently operate the facilities.
The captured biogas may be treated and sold to a natural gas utility or used as fuel for fleet vehicles. Captured biogas can also be burned to generate electricity or heat, ideally in a combined heat and power system.
Reducing Metallurgical Coke Production for Iron and Steel
Most iron and steel is created in blast furnaces or basic oxygen furnaces. These furnaces are used to convert mined iron ore into pig iron or to convert pig iron and various alloy metals into steel. Blast furnaces are fueled by coke, a substance created when pulverized coal is heated in an oxygen-free environment, a process called coking. Coke is used both as a chemical-reducing agent (to remove oxygen from the iron oxide in iron ore) and to generate the high temperatures needed for steelmaking.
Several techniques exist for reducing coke production and use. Electric arc furnaces can generate the high temperatures needed to make steel without the need for coal, but they do not provide a source of carbon as a chemical-reducing agent, so they are used primarily to reuse scrap steel rather than to create new steel.
Modern blast furnace designs can reduce the amount of coke needed to produce a quantity of steel. And steel companies are developing technology that would allow natural gas to be used in place of coke as the carbon source in steelmaking, thereby offsetting the emissions generated when producing coke.
Policies to Reduce Process Emissions
Monitoring and reporting requirements may be a necessary precondition for the application of certain other policies, such as performance standards, financial incentives, or a carbon price. Currently, few industrial facilities measure or track their process emissions. Where direct measurement may not be practical (e.g., in agricultural operations), equations may be developed that estimate process emissions based on the amount and type of activity taking place (e.g., the number of cattle in a dairy, what they are fed, how their manure is managed).
Performance standards may be most familiar in the context of regulating energy use (such as the distance a car can travel on a given quantity of gasoline), but they can also be applied to process emission sources. For example, government may mandate that a certain percentage of landfill gas be captured from landfills or that no more than a certain percentage of clinker is used in cement.
In the natural gas and petroleum industry, standards may mandate green well completions, which reduce methane leakage at the wellhead. Coal mines may be subject to requirements for capture and flaring of methane. To control F-gases, governments may ban specific refrigerants and propellants. Standards may also be used to tackle building quality and longevity, lowering the amount of cement needed for reconstructing buildings.
A carbon price via a carbon tax or cap-and-trade program can provide a financial incentive for companies to find cost-effective ways to reduce process emissions. Of course, to be effective the carbon price must be applied not only to energy-related emissions but also to process emissions, and it must be levied not only on CO2 but also on other greenhouse gases.
Financing assistance can help companies purchase different or upgraded equipment, such as an anaerobic digester for a dairy, or retool their assembly lines to use substitutes for F-gases in refrigerators and air conditioners. Financing could be provided via grants, low-interest loans, loan guarantees, a revolving fund, a green bank, or other mechanisms.
Government procurement decisions can account for lifecycle emissions, including process emissions. If government refuses to purchase goods whose manufacture entailed high emissions (or applies a “shadow price” to these goods, making them less able to compete with goods produced in a more environmentally friendly way), this encourages emission cuts by companies that want to sell their goods to the government. An example of this type of policy is California’s Buy Clean Act.
Technical assistance can help some businesses, particularly small and medium enterprises and farmers, to understand their sources of process emissions and mitigation strategies. Information about best practice inspection and maintenance protocols can help avoid degradation in performance over time. Technical assistance may be particularly helpful in developing countries and when dealing with industries containing many small producers, such as agriculture (livestock measures, cropland management), and in some countries, the cement and waste management industries.
Economic signals for goods or materials substitution may be used to divert purchases from high-emission to lower-emission choices. For example, a tax on virgin steel or a subsidy for recycled steel may increase the share of steel production from scrap steel in electric arc furnaces. A tax on meat from ruminant animals may encourage a shift to non-ruminant animal or plant sources of protein. And a variety of policies can encourage a shift from coal-fired power generation to other electricity sources.
When to Apply This Policy
Because almost every country has agricultural or industrial production, it is likely that almost every country could benefit from application of some of these policies. The choice of policies to focus on in a particular country should be guided by the prevalence of relevant industries and practices that could be changed.
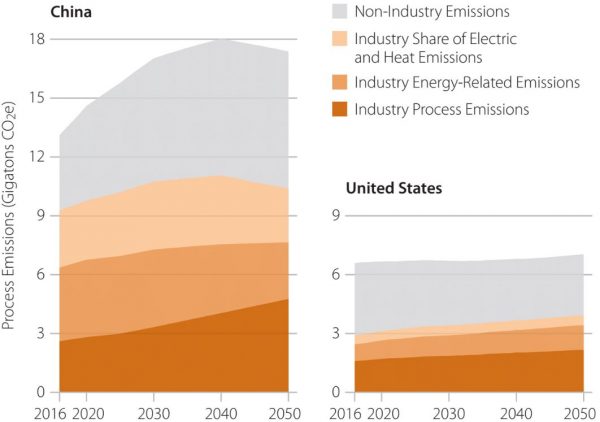
U.S. and China business-as-usual process emissions in context.
For example, in countries with rapid urbanization and building quality problems, such as China, codes mandating building and infrastructure quality, to promote longevity, may be among the highest-priority policies. It is cost-effective to put strong building codes in place while a country is still expanding its infrastructure base and building stock, to avoid the cost and emissions associated with rebuilding. In a country that is already urbanized and has a high-quality building stock, these policies are less important.
Countries with large amounts of natural gas production and old gas distribution infrastructure, such as the U.S., are prime targets for policies tackling methane leaks. Countries with large coal mining operations should consider ways to tackle coal mine methane. Any country that produces refrigerants and propellants should use policy to mandate a transition from F-gases to safer chemicals.
Case Studies
The Montreal Protocol
The Montreal Protocol is a landmark international agreement finalized in 1987 and amended several times since then, most recently in Kigali in 2016. In this treaty, countries agreed to phase out ozone-depleting substances that were progressively destroying Earth’s stratospheric ozone layer, which protects the surface from harmful radiation. The treaty was successful at reducing emissions of more than 100 ozone-depleting substances by more than 99%.
The protocol is widely regarded as the most successful international environmental treaty. In fact, United Nations secretary-general Kofi Annan stated that it was “perhaps the single most successful international agreement to date” (i.e., environmental or otherwise). It was the first universally ratified treaty in United Nations history.
Originally, the Montreal Protocol primarily targeted two categories of gases: chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), both of which damage the ozone layer and are also potent greenhouse gases. CFCs, the more harmful of the two categories of gases, were to be phased out on a faster schedule than HCFCs. The protocol was amended several times to add new chemicals to the list of controlled substances and to accelerate the phase-out timetables for chemicals already on that list.
In 2016, under the Kigali Amendment, the agreement was modified to require the phase-out of hydrofluorocarbons (HFCs). This class of chemicals is not damaging to the ozone layer, but HFCs are potent greenhouse gases, and they are used for some of the same purposes (such as refrigerants and aerosol propellants) as were CFCs and HCFCs before them. This amendment, and actions taken by individual countries to comply with it, may be the best policy option for reducing F-gas process emissions globally.
Reasons for Success
There are several reasons for the Montreal Protocol’s success. First, the science behind ozone layer depletion was widely accepted by the general public. After the 1973 discovery that CFCs could lead to ozone depletion, the industries that manufactured CFCs and aerosols, particularly DuPont, undertook a massive disinformation campaign in an attempt to cast doubt on the link between their products and ozone layer damage. However, the discovery of the ozone hole above Antarctica in 1985 helped to confirm the science and demonstrate the urgency of the problem, catalyzing the international community.
Second, the Montreal Protocol included a multilateral fund, which provided a way for developed countries to provide financial support to help developing countries transition away from CFC and HCFC use. This fund is still important today in facilitating the phase-out of the last HCFCs still being produced in a few developing countries.
Third, the treaty avoided becoming politicized. In the U.S., it was Republican president Ronald Reagan’s crowning environmental achievement, and it has been supported and strengthened by both Republican and Democratic presidents.
Examples of Country-Level Implementation: The United States and Japan
As an international treaty, the Montreal Protocol had to be made effective through laws in each individual country. This is where the policy design guidelines in this section come in.
For example, the U.S. Environmental Protection Agency (EPA) implemented the HFC phase-out through the Significant New Alternatives Policy program. Under this program, the EPA evaluated alternative chemicals for a variety of different use cases, such as chemicals used for fire suppression, household refrigerators, and motor vehicle air conditioning systems. Factors considered by the EPA include effects on ozone depletion, climate change, exposure and toxicity to humans, flammability, and other environmental impacts. The EPA uses a comparative risk framework: It does not require that substances be risk-free, and it restricts only chemicals whose impacts are significantly worse than alternatives, to avoid interfering with the market’s selection of alternative substances more than necessary.
Japan has been a leader in using policy to accelerate the transition away from F-gases. Even before the Kigali Amendment, Japan enacted policies to begin drawing down refrigerant emissions. Japan’s law, enacted in 2015, covers the entire product lifecycle. It phases down refrigerant production by manufacturers and importers, promotes low–global warming potential and non-fluorocarbon products, prevents leakage from equipment while in use by requiring periodic checks and maintenance, and promotes recycling by licensed companies at end of equipment life.
Japan worked with 14 domestic industry organizations, such as the Japan Chemical Industry Association and the Japan Automobile Manufacturers Association, to implement product labeling with refrigerant and global warming potential information and to encourage manufacturers to set and achieve voluntary targets.
Zimbabwe’s National Domestic Biogas Program and California’s Dairy Digester Research & Development Program
Of the process emission reduction technologies discussed in this section, anaerobic digesters for manure and organic waste are among the most broadly applicable. Government programs to fund digester construction exist in places ranging from California, one of the world’s most developed and high-tech economies, to rural Zimbabwe. Both of these programs are discussed here to illustrate the range of approaches that can be used to successfully deploy this technology.
In California, the state’s Department of Food and Agriculture operates the Dairy Digester Research & Development Program, which provides financial assistance for the installation of digesters in dairies in California. The program is funded by the state’s Greenhouse Gas Reduction Fund. In 2017, 18 projects were awarded a total of $35.1 million in funding, with a total project cost of $106.4 million, so every government dollar leveraged more than $3 in private sector money.
Project sponsors must submit a detailed application and are carefully evaluated against a variety of criteria, including benefits to disadvantaged communities, financial strength, greenhouse gas emission reduction estimates, economic and environmental co-benefits, and project readiness (including compliance with the California Environmental Quality Act and other laws, as well as outreach to surrounding communities). Awardees must re- port verified emission reductions for 10 years after the project is operational.
In Zimbabwe, three government ministries partnered with two Dutch nongovernment organizations, SNV and Hivos, to create the National Domestic Biogas Programme. This program aims to construct digesters throughout Zimbabwe to facilitate the creation of biogas (a mixture of gases from decomposition of organic material, primarily methane).
Rather than being used to generate electricity, as in California, the biogas created in Zimbabwe will be used directly for cooking and lighting and for powering biogas-burning appliances. Anaerobic digesters have many benefits when used in rural Africa. In addition to reducing emissions, a digester may improve the sanitary conditions on a farm, eliminate indoor air pollution from the burning of traditional biomass, significantly reduce the workload related to food preparation, and produce high-quality fertilizer (the fermented bio-slurry).
However, anaerobic digesters need significant amounts of manure and water to operate effectively, so they may not be a fit for parts of Africa that lack access to reliable sources of usable water. To date under this program in Zimbabwe, more than 70 masons and 18 fabricators of parts have been trained in how to construct biogas plants. The project has supplied more than 1,385 households with biogas, has a target to construct 8,000 digesters by 2018, and aims to establish a vibrant, local biogas sector that will ultimately serve more than 67,000 households.
U.S. Regulations to Reduce Methane Leaks from The Natural Gas and Petroleum Industry
In 2012, the EPA enacted standards limiting the emissions of volatile organic compounds, a type of local air pollutant, from natural gas and petroleum operations. In 2016, this rule was extended to cover greenhouse gases, particularly methane, and to cover a greater range of industrial activities and equipment. The new rule also requires owners and operators to find and repair methane leaks.
The rules require monitoring for leaks on a fixed schedule (twice a year or quarterly depending on the type of equipment). The rules allow multiple leak detection methods, including optical imaging or the use of an organic vapor analyzer, and they permit producers to apply to use other technologies, to allow innovation.
The rule also phases in requirements that drillers perform green well completions. After a new well is drilled, before it begins production, a substance called flowback (a mixture of drilling fluids, gas, oil, water, and mud) is extracted from the well. This process takes from a single day to several weeks. (Wells involving hydraulic fracturing, or “fracking,” produce more flowback than non-fracked wells.)
In a conventional well completion, the flowback is directed to an open pit or tank, where the gas released from the mixture is vented to the atmosphere, or sometimes flared. In a “green completion,” equipment is used to separate the gas, liquid, and solid components of the flowback mixture, and the gas is captured for onsite use or sale. The necessary technology is mature and can capture up to 90% of the gas in the flowback stream.
The EPA has estimated that the rule will yield climate benefits of $690 million in 2025, outweighing the costs of $530 million, and will also achieve improvements in public health (reduced illness and deaths) thanks to reductions in toxic air pollutants.
Conclusion
Industrial process emissions are an important source of greenhouse gases, accounting for 55% of all industry-related emissions in the U.S. and 28% in China. Although industry is diverse, just nine types of activity produce almost 90% of global process emissions: enteric fermentation and manure from livestock; methane leaks from natural gas systems; cement clinker production; emissions from soils, rice cultivation, and fertilizer use; methane produced in landfills; refrigerant use; methane leaks from coal mines; methane from wastewater treatment; and metallurgical coke for iron and steel production.
Techniques exist to tackle each of these types of emissions, and policies such as monitoring and reporting requirements, performance standards, carbon pricing, and financial and technical assistance can help achieve emission reductions. Examples of successful programs include the Montreal Protocol (which in 2016 was expanded to cover F-gases) and anaerobic digester deployment initiatives in California and Zimbabwe.